These nutrients are required only in extremely small quantities and are often described as "trace elements." They play important roles in the biochemical processes that sustain life and are vital for good plant health.
Boron
Boron is absorbed by plants in the form of borate (BO33-) and is required for cellular membrane function, root growth, carbohydrate transport, metabolic regulation, and flower production. Aquatic fertilizers often contain boron in the form of borate or borax (sodium borate) and it is also present in most tap water sources. Normally, boron deficiency is unlikely to occur in the aquarium and the nutrient is not considered a vital additional source of fertilization.
Iron
Iron is an important micronutrient used in respiration, enzyme production, and chlorophyll synthesis. Plants will absorb iron both through their roots and leaves. As a nutrient, iron is most useful to plants in the ion form Fe2+, although in the presence of oxygen it converts into Fe3+, which is difficult for plants to assimilate. This problem can be overcome by using chelates or chelated iron. (Chelates are dissolved organic substances that bind to metals and prevent them from forming larger molecules through oxidation.) The most common chelate used in fertilizers is EDTA, which is often used to supply chelated iron (FeEDTA). Fe2+ is then slowly released by the chelated iron and becomes available to plants. Although iron — or, more precisely, chelated iron — is heavily promoted as a vital plant fertilizer, there is a plentiful supply in soil-based or nutrient-rich substrates. Iron and natural organic chelates, combined with a low-oxygen substrate, ensure that iron is always present in an available form. Most aquatic fertilizers contain iron and chelates and should always be used in aquariums without soil-based substrates.
Chlorine
 Chlorine is absorbed by plants in the form of chloride (Cl-) ions and is used for osmosis, ionic balance, and also in photosynthesis. Chloride is normally present in sufficient quantities in tap water (even after the use of dechlorinators), so should not present any nutritional problems for plants.
Chlorine is absorbed by plants in the form of chloride (Cl-) ions and is used for osmosis, ionic balance, and also in photosynthesis. Chloride is normally present in sufficient quantities in tap water (even after the use of dechlorinators), so should not present any nutritional problems for plants.
Nickel
Aquatic plants use nickel as an ion (Ni2+) in extremely tiny amounts in the production of the enzyme urease (which breaks down the nitrogenous compound urea into ammonia). It is normally present in tap water and soil or in nutrient-based substrates. Nickel deficiencies or excesses should not occur under the vast majority of aquarium conditions.
Copper
Plants absorb copper as an ion (Cu2+), both from the water and the substrate, although humic acids and organics in the substrate often bind with copper and other metals, making them unavailable for plant uptake. Copper is a key part of enzymes that facilitate respiration, but is only needed in tiny amounts by plants. Additional fertilization with copper is not required. Indeed, in most cases, tap water contains far more copper than plants require, but since they have little or no control over how much they absorb, they simply take up whatever is available. If they absorb too much, the result is metal toxicity, typically resulting in brown spots and tissue breakup. Copper is occasionally used in treatments to control parasites or algae, so use these with care. The maximum safe level of copper in water is much higher for humans: 1.3 ppm (parts per million, equivalent to mg/liter) than for fish: 0.02 ppm. For this reason, plants offer themselves as a vital resource for reducing copper concentrations in the aquarium, which may be at dangerous levels for fish if the water is sourced from tap water. Plants are sometimes used exclusively for the purpose of removing copper and other dangerous metals.
Manganese
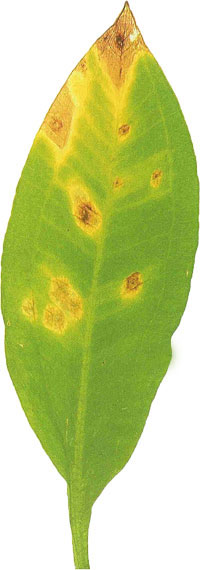 Manganese is absorbed as an ion (Mn2+) through the roots and leaves of aquatic plants. It activates enzymes used in chlorophyll production and photosynthesis. Plants require relatively low levels of manganese, but it is still an important micronutrient. In most cases, manganese is present in tap water in sufficient quantities, but fertilizing with a liquid fertilizer will ensure that plants do not suffer a manganese deficiency.
Manganese is absorbed as an ion (Mn2+) through the roots and leaves of aquatic plants. It activates enzymes used in chlorophyll production and photosynthesis. Plants require relatively low levels of manganese, but it is still an important micronutrient. In most cases, manganese is present in tap water in sufficient quantities, but fertilizing with a liquid fertilizer will ensure that plants do not suffer a manganese deficiency.
Molybdenum
Molybdenum is an important nutrient for aquatic plants. It is a component of an enzyme used by plants to reduce nitrates (NO3-) into ammonium (NH4+) for protein synthesis, and is especially important in hard water conditions, where little or no ammonium is available as a source of nitrogen. Plants absorb molybdenum in the form of molybdate (MoO42-). This is available in tap water, but additional fertilization with substrate, tablet or liquids will ensure sufficient levels in the aquarium.
Zinc
Zinc an important nutrient for overall plant health, is a component of many enzymes and is involved in chlorophyll formation. Zinc is taken up in its nutrient form (Zn2+) through the leaves and roots. At high concentrations it is toxic to both fish and plants, although sensible use of liquid fertilizers should ensure that these do not occur. Traces of zinc at levels high enough for aquatic plants are present in tap water, liquid fertilizers, and nutrient-rich substrates.
Nutrient deficiencies and excesses
An excess or deficiency of one or more nutrients can cause problems to both fish and plants in the aquarium. Because plants have no control over the amounts of some of the nutrients they absorb (such as copper) excesses can cause fundamental problems within individual cells that in turn affect the entire plant. Providing a correct balance of nutrients without supplying too much or too little of any particular nutrient is very difficult. The levels of most nutrients cannot be easily determined and the only way to judge whether there is a nutrient problem is to observe the health of the plants. Deficiencies can be spotted in many ways, mainly in the form of slow or deformed growth, alterations in the color of leaves, or a breakdown of cell structure. They may only affect some plants due to differences in individual nutrient requirements. Sometimes, a deficiency of one nutrient can be caused by an excess of another. In this instance, the nutrient present in excess "competes" with the second nutrient preventing it from being absorbed by the plant and causing a deficiency. A good indicator of whether a particular problem is caused by an excess or a deficiency of a nutrient can be seen in the difference in symptoms between slow-growing and faster-growing plants. If there is an excess of a certain nutrient faster-growing plants may not be affected because they can essentially 'dilute' the nutrient through fast production of new leaves. Slow-growing plants have no choice but to increase their buildup of the nutrient until signs of excess begin to show The same process works in reverse; faster-growing plants will show signs of nutrient deficiencies before sloW-growing plants, which have higher reserves. Plants will generally take up more nutrients than they need and simply store them in the cell tissue for later use. Problems occur when the storage capacity of a plant is exceeded and the nutrient buildup becomes toxic, affecting the fun. ction of cells. For example, a buildup of iron manifests itself in the form of brown spots on the leaves. These are literally deposits of iron that have collected in one area. Over time, further deposits prevent the cells in that area from functioning properly and the affected area dies back. Many nutrient problems take the form of yellowing of the leaf tissue - chlorosis - caused by a lack of chlorophyll. Chlorosis occurs when a plant is unable to produce normal amounts of chlorophyll due to a lack of nutrients. Since chlorophyll is used for photosynthesis, a lack of it prevents this vital process and affects every aspect of a plant's health. Many nutrients are.used in the production and correct functioning of chlorophyll, so chlorosis can be used as a general indicator of nutrient problems in the aquarium.


